Abstract
Follicular lymphoma (FL), a malignancy derived from germinal center B cells, is characterized by ongoing somatic hypermutation of the immunoglobulin variable region (IGV) genes. This somatic hypermutation can introduce novel N-glycosylation sites (N-sites), consisting of Asn-X-Ser/Thr motifs, in the IGV resulting in the addition of glycans at these sites. Binding of lectins, such as DC-SIGN, to these glycosylated surface immunoglobulins (i.e., B cell receptors) activates the B cell receptor pathway in an antigen-independent manner, promoting FL B cell survival and proliferation. Previous studies have concluded that the acquisition of N-sites is a stable and clonal event during FL evolution (Odabashian et al., Blood 2020). Here, we investigated the dynamics and potential impact of N-site prevalence at a single cell level, in paired heavy and light chains, across sites and over time in 16 patients with FL.
We profiled tumor fine needle aspirates from patients enrolled in two clinical trials (NCT02927964, NCT03410901). 15 of 16 patients were previously, but not recently treated, while one was previously untreated. Samples were collected from different tumor sites before treatment, on-treatment, and at tumor progression. Single cell suspensions were subjected to single cell B cell receptor (scBCR) and RNA (scRNA) sequencing using the 10X chromium platform. Only BCR sequences belonging to patient-specific dominant malignant heavy and light chain clones were considered for further analysis.
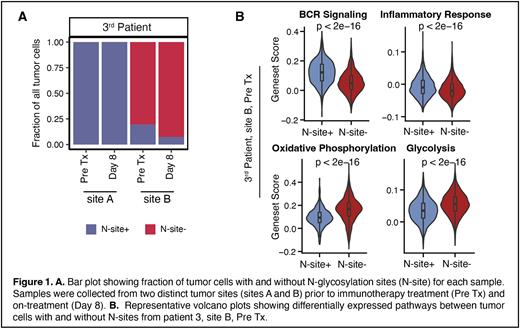
In total, we analyzed more than 250,000 FL tumor cells from 89 tumor samples from 16 FL patients. While most patients acquired at least one N-site as an early and stable event that was conserved across distinct tumor sites and over time, 3 patient cases proved to be exceptions: one patient had no detectable N-sites in either the heavy or light chain IGV genes at any tumor site or timepoint. In a second patient, only one of two tumor sites exhibited a novel N-site, and this was probably acquired after divergence of the tumor populations to distinct tumor sites (Haebe, Shree et al., Blood 2021). Interestingly, clinical tumor progression in this patient arose from selection and expansion of an N-site negative subclone, despite the putative survival advantage conferred by acquired N-sites. In the third patient, N-sites were acquired in a precursor cell before subclonal divergence, but subsequently overtaken by an N-site-negative subpopulation, which comprised up to 90% of tumor cells at a given site (Figure 1A). These results suggest that other acquired features can out-compete the selective advantage of N-glycosylation. To elucidate biological differences between cells with and without N-sites within each sample, we integrated scBCR and scRNA data for the third patient using shared cell barcodes. N-site positive cells exhibited a higher expression of the BCR pathway and inflammatory response, while cells without N-sites had higher activity of metabolic processes, including oxidative phosphorylation and glycolysis (Figure 1B).
In conclusion, while acquired N-sites likely support FL pathogenesis through antigen-independent BCR signaling in many FL patients, selection and expansion of N-site negative cells in some patients in our study suggests that N-site negative cells can thrive, and likely depend on other pathways, with our preliminary results pointing to differential metabolic regulation.
Disclosures
Shree:Gilead Sciences: Other: Spouse's employment. Long:Lunit: Consultancy. Levy:Abintus Bio: Consultancy; Kira Pharmaceuticals: Consultancy; Walking Fish Therapeutics: Consultancy; Immunocore: Consultancy; Spotlight Therapeutics: Consultancy; Viracta Therapeutics: Consultancy; Apexigen: Consultancy; Dragonfly: Consultancy; Nurix: Consultancy; Teneobio: Consultancy; GigaGen: Consultancy; BeiGene: Consultancy; Quadriga BioSciences: Consultancy; Khloris Biosciences: Consultancy; Virsti Therapeutics: Consultancy; BioLineRx: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal